Organometallic Solutions: #3
3.* (1996 2 2) For each of the following diamagnetic, coordinatively saturated ("18 electron") complexes: 1. Identify the metal, Note that the nature of the metal (3d, 4d, or 5d) is specified. A. M(CO)4 (3d) CO is a neutral sigma base and a p acid, 2 electron
donor. B. (C6H6)2M+ (5d) The ligand is benzene, which is a neutral 6-electron donor. The complex is a "sandwich molecule":
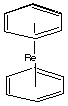
1. Re
C. [Cp2M] (3d) The ligand is cyclopentadienyl, which is a 6 electron donor with a -1 charge. The complex is a "sandwich molecule":

1. Re
|