|
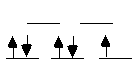
[IrCl6]2-
This is an Ir[IV] complex which is d5. It's low
spin (Ir is a 5d transition metal; all 4d and 5d metals are low spin)
and has octahedral splitting. This complex wants another electron, so
it wants to oxidize and be reduced. [IrCl6]2- is a better
oxidizing agent. |
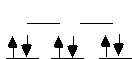
[PtCl6]2-
This is a Pt(IV) complex which is d6. It's low spin and HAPPY with no unpaired electrons. Adding another electron will destabilize this complex because the electron will be forced to go into the antibonding eg orbitals. [PtCl6]2- is not a good oxidizing agent. |