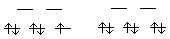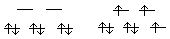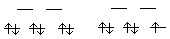Organometallic Solutions: #14
14.* (1993 3 2) Which of the following reactions do you expect to be the fastest; which the slowest. Explain your answer. (1) [Ru(dipy)3]2+ + [Ru(phen)3]3+ --> These are all outersphere e- transfers. The less that nuclear rearrangement of the ligands (geometry change in bond lengths) is required, the less the Ea will be and the faster the reaction. So t2g --> t2g e- transfers will be fastest. [Ru(dipy)3]2+ + [Ru(phen)3]3+ --> t2g --> t2g
[Co(en)3]3+ + [Co(en)3]2+ -->
eg --> eg
[Ru(NH3)6]2+ + [Ru(NH3)6]3+ --> t2g --> t2g B. Copper(I) disproportionates into copper(II) and Cuo (Eo Cu+/Cuo = +0.52 V; Eo Cu2+/Cu+ = +0.15 V). Given the following Eo values for Ag, calculate the feasibility of Ag+ disproportionating into Ag2+ and Ago. (Eo Ag+/Ago = +0.799 V; Eo Ag2+/Ag+ = +1.98 V). If everything is in standard states (temperature and pressure at STP,
everything aqueous having 1M concentration), E = Eo. Therefore, we can use Eo > 0 as the criteria for a spontaneous reaction.
From the given potentials, we can write the following:
Ag+ + e- --> Ago Eo = +0.80 V Eo < 0 so this disproportionation is nonspontaneous. Instead, the reverse, "comproportionation" is spontaneous. Ago + Ag2+ --> 2Ag+ Eo = +1.18 V |