Organometallic Solutions: #13
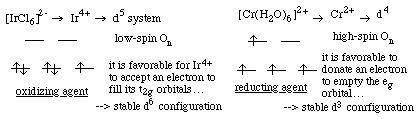
13.* (1993 3 1) Explain each of the following. A. Metal containers corrode more rapidly in dilute HCl than in aqueous H2SO4 solutions having the same pH. Corrosion is an oxidation process… i.e. it involves electron transfer from the metal to an oxidizing agent. Cl- can act as a bridging ligand between the metal and the oxidizing agent. This will increase the rate of corrosion relative to H2SO4. B. The anion [IrCl6]2- is a strong oxidizing agent whereas the cation Cr2+ aqueous is a strong reducing agent.
C. At the time Stanford was founded, aluminum metal dinner plates were more expensive than solid silver plates whereas today the opposite is true. Look at Eo
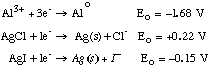 From natural sources, oxides and/or salts, reducing Al3+ to Alo will be more difficult since it is highly thermodynamically unfavorable… Not true for Ag salts. It was difficult to find reducing agent with Eo +1.68 V until electrolysis was perfected ~1900 A.D. D. A sample of [Co(dipy)3](ClO4)3, A, in aqueous solution has been resolved and is optically active. The optical isomer is stable for prolonged periods at room temperature. All attempts to resolve [Co(dipy)3](ClO4)2, B, failed to yield an optically active product. When a trace of B was added to a solution of optically active A, the optical activity of the latter slowly fell to a value of zero. Discuss both the mechanism and the thermodynamic driving force for this reaction. Outersphere electron transfer…
For the outersphere mechanism: DH = 0, DS => (+) positive (racemization causes more disorder). The driving force for the reaction is an increase in entropy. |