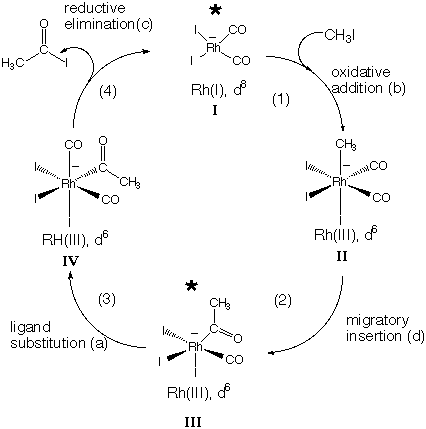
Organometallic Solutions: #11
11.* (1994 2 4) The following is a list of reaction classes which encompasses the majority of the transformations of organometallic compounds: (a) ligand substitution or dissociation, (b) oxidative addition, (c) reductive elimination, (d) migratory insertion, (e) electrocyclization. Now consider the catalytic cycle of reactions which are known to occur in the Monsanto acetic acid process in which methanol combines with CO to form acetic acid: CH3OH + CO ---(Rh catalyst, I-)--> CH3CO2H
The steps in this cycle are labeled (1), (2), (3), and (4); the intermediate complexes are labeled I, II, III, and IV. Over the arrow for each step put the letter from the above list indicating the nature of that step. Under each intermediate complex put the formal oxidation state and d electron configuration of the central metal (i.e. Mo(II) d4). Place an asterisk beside each coordinatively unsaturated ("16-electron") complex. The diagram is shown below, with the oxidation states and d electron configurations of the metals, as well as the type of reaction involved (oxidative addition, reductive elimination, etc.) Coordinatively unsaturated (16-electron) complexes are marked with an asterisk. |