Organic Chemistry Solutions: #8
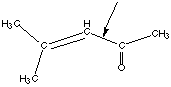
8. Consider the molecule shown below.
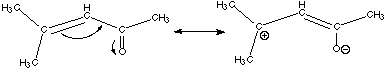
A. Briefly explain why rotation about the labeled carbon-carbon bond is hindered. This molecule has a resonance structure of the kind shown below:
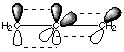
Rotation about double bonds is impossible, so the rotation of the full structure is hindered. This is an example of what is known as a conjugated system. It is especially stable because each of the atoms involved has a parallel pi orbital. The electrons can delocalize through the entire pi system, and delocalization gives rise to stabilization. B. Briefly explain why cumulated systems (such as allene) are inherently unstable. Allene has two adjacent double bonds. The only way this can happen is to have the central carbon sp hybridized with one p orbital forming a pi bond to one carbon and the other perpendicular p orbital forming a pi bond to the other carbon. No delocalization through the pi system is possible in such an arrangement. Moreover, the pi orbitals on the central carbon are 90 degrees apart from each other, which is unpleasant for the mutually repulsive electrons. You will recall that sp3 orbitals are 109.5 degrees apart from each other, which is much roomier for the electrons. This unnatural crowding the of the like-charged electrons by makes cumulated systems such as allene inherently unstable.
|