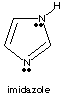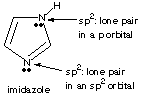Organic Chemistry Solutions: #3
3.* (1997 1 2) Below is a line drawing of imidazole, an aromatic heterocycle found in the amino acid histidine.
A. Draw an arrow to each nitrogen and indicate the hybridization state (sp3, sp2, or sp) for that nitrogen. see below B. Each nitrogen has an electron pair. For each nitrogen indicate the nature of the orbital containing that electron pair.
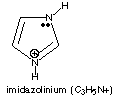
C. Explain the origin of imidazole’s aromaticity. Imidazole has 6 p electrons in a closed cycle. The top right N contributes 2; each other atom contributes 1 electron via its unhybridized p orbital. You could also count the p electrons by taking 2 for each p bond and 2 non-bonded p electrons. D. Imidizole is a Brønsted base; protonation of imidizole forms a cation, C3H5N+. Complete the partial structure of the cation, showing line bonds and electron pairs.
Note that imidazole is still aromatic upon protonation--if it lost its aromaticity it probably wouldn’t be a Bronsted base! |