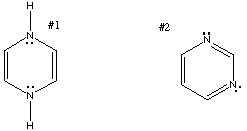
Organic Chemistry Solutions: #24
24.* (1991 1 3) Of the following two structures, which is more stable? Why? For #1, the symmetry of the molecule dictates that the lone pairs on the two nitrogens must be in the same type of orbital. Either both lone pairs are in p orbitals (8 pi electrons) or they are both in sp3 orbitals (4 pi electrons). In the former case, the molecule is antiaromatic due to its 4n pi electrons. In the latter case, the molecule is non-aromatic because not all of the atoms in the ring have p orbitals. For #2, each nitrogen is sp2 hybridized. The lone pairs of the nitrogens are in sp2 hybrid orbitals. The p orbital of each nitrogen contains one pi electron, as does the p orbital of each of the carbons. Therefore, the structure has a total of 6 pi electrons and is consequently aromatic. |