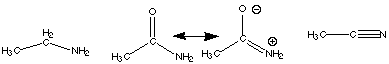Organic Chemistry Solutions: #20
20.* (1992 1 3) Consider the following 3 molecules: CH3CH2NH2, CH3CONH2, CH3CN. A. Which one has the longest carbon-nitrogen bond? Explain, using hybridization arguments.
The CH3CH2NH2 has the longest C-N bond because both C and N are sp3 hybridized and are connected by a single s bond only. In CH3CONH2 , nitrogen is sp2 hybridized so that its lone pair can participate in the pi system of the carbonyl. The C-N bond is stronger than a single bond and is therefore shorter. In acetonitrile, nitrogen is sp hybridized and is connected to carbon through one s bond and two pi bonds. The formation of three bonds requires that the carbon and nitrogen approach each other to allow their orbitals to overlap more effectively.
|