Organic Chemistry Solutions: #18
18.* (1993 1 4) A. What is meant by an "antiaromatic" compound? Write a standard structural formula for any example of such a substance. An antiaromatic compound does not obey the Hückel rule (4n+2 pi electrons in a closed cycle with a p orbital on every atom) and does not exhibit resonance stabilization. Unlike aromatic compounds, an antiaromatic compound is not flat, because to be flat would necessitate putting electrons in the high-energy antibonding orbitals of the aromatic configuration. To avoid this, the antiaromatic compound is typically buckled into a boat shape like cyclo-octatetraene, below:

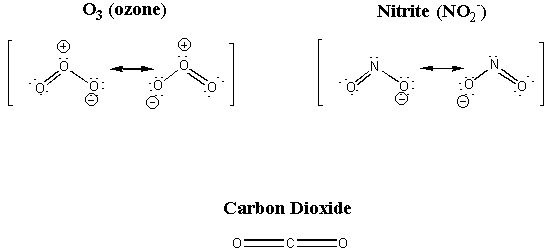
B. Two of the following three substances have something in common which is reflected in their structures. Can you identify this commonality? Propose structures for all three: O3, CO2, NO2-.
O3 and NO2- both have a bent structure and are isoelectronic. Carbon dioxide is the odd one out, being linear and having 14 electrons.
|