Organic Chemistry Solutions: #17
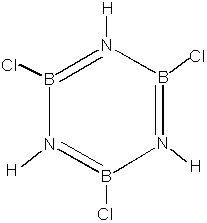
17. Consider the molecule of borazine shown below.
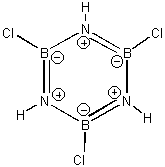
A. Label the formal charges and hybridization states of all of the atoms in borazine.
Note that all atoms in the ring (nitrogens and borons) are sp2. B. Where do the electrons in borazine's pi electron cloud come from? Is borazine aromatic? Each nitrogen donates two electrons to borazines pi electron cloud. Boron doesn't donate any, but it has an empty p orbital available through which the electrons can delocalize. With 6 p electrons (2 from each nitrogen) borazine is aromatic. C. What stable resonance structures are possible for borazine? 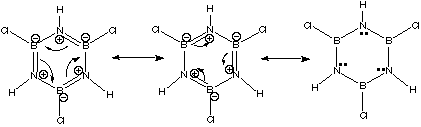 In the above pictures, each curved arrow represents the flow of two electrons. The structure on the far right has the electrons in the "double bonds"; all localized in the nitrogen p orbitals. D. Which would be more stable: borazine or benzene? Briefly explain your answer. Borazine is made up of boron (electropositive: Lewis acid) and nitrogen (electronegative: Lewis base). Therefore, its pi electron cloud is "lumpy," with the electrons spending more time near the nitrogens than near the borons. Note that although the electrons in the cloud spend more time near the nitrogens, the nitrogens have a positive formal charge! This should reinforce your understanding of formal charge as a book-keeping system only. It does give us valuable insight; nitrogen does get less electron density than it would like for having to share. Still, though, its natural electronegativity assures that it will get the lions share of the pi cloud. We can see this from the resonance possibilities: one of the resonance structures has the electrons fully localized on nitrogen. Since benzene’s electron cloud is more fully delocalized, and since electron delocalization is what gives aromatic molecules their special stability, benzene would be more stable than borazine. |