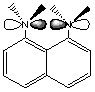Organic Chemistry Solutions: #14
14. A molecule that is known as a "proton sponge" is pictured below. Note that the lone pairs on the nitrogen atoms are in orbitals that are in the plane of the molecule.
A. Is the proton sponge aromatic? Yes, there are 10 electrons in the pi system. 4(2) + 2 = 10, so the system obeys Hückel’s rules. B. Why does this molecule suck up protons so well? Protons can be optimally sandwiched between the two nitrogen lone pairs. C. Do you think the proton sponge would be as effective if it had protons replacing its methyl groups? Why or why not? Methyl groups provide a steric hindrance to rotation about the bond between the ring carbon and the nitrogen. This forces the orbitals containing the lone pairs to stay in orbitals facing each other. If there were hydrogens in place of the methyl groups, the bond could rotate, aligning the nitrogen lone pair orbitals with the pi system of the molecule. The lone pairs would then be farther from each other and could resonate into the ring. This would make them less likely to want to suck up protons. |