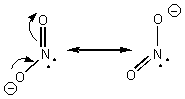Organic Chemistry Solutions: #11
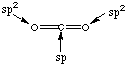
11. Draw resonance structures for the following molecules. Label the hybridization states and formal charges for each structure you draw. A. Carbon dioxide (CO2)
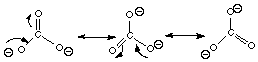
B. Carbonate ion (CO32-)
All of the atoms in the carbonate molecule are sp2 hybridized, regardless of which resonance structure you’re talking about. Each atom has to have a p orbital available to promote electron delocalization. C. Nitrite ion (NO21-)
All of the atoms are sp2 hybridized. The nitrogen lone pair is in an sp2 hybridized orbital. D. Ozone (O3)
All of the atoms are sp2 hybridized. The negative charge is split evenly between the outer two oxygen atoms, while the positive charge remains localized in the central atom in both resonance structures. |