Organic Chemistry Solutions: #10
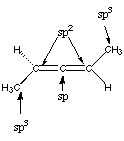
10.* (1995 1 3) Consider a hydrocarbon X, (molecular formula C5H8), which is chiral. This substance manifests all three hybridization states of carbon. Reaction of X with H2 over a Pd metal catalyst forms n-pentane. Such reactions do not break carbon-carbon bonds. A. How many sites of unsaturation are in X?
B. Draw a three dimensional structural formula for X.
Notice that this molecule doesn’t have any improper rotation axes (notably a mirror plane or an inversion center), which is the group theoretical criterion for chirality. Be careful with this one, though! It almost has an S4 axis! However, you will see that if you rotate the molecule so that the right side reflects into where the left side used to be, then the left side won't reflect into where the right side used to be. Click here for an interactive picture of allene that might help you see this . . . |