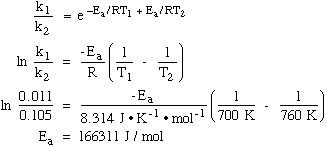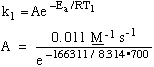Kinetics Solutions: #9
9.* (1994 F 5) The thermal decomposition of acetaldehyde (CH3CHO) was measured at two temperatures. At T=700K, the rate of this second-order process was 0.011 (mol/L)-1s-1and at T=760K it was 0.105 (mol/L) -1s-1. Use this data to estimate the activation energy Ea and the pre-exponential factor A given that the universal gas constant R has the value 8.314 JK-1mol-1. State clearly the units of Ea and A. Arrhenius equation:
(1) (2)
Use state 1 data to calculate A:
|