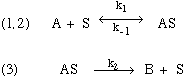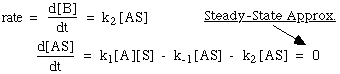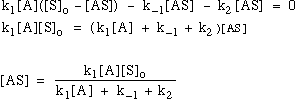Kinetics Solutions: #8
8.* (1994 F 4) Metal surfaces often serve as catalysts. For example on a hot copper surface, ethanol (C2H5OH) decomposes to acetaldehyde (CH3CHO) and hydrogen (H2). Experimentally, it is found that at low concentrations of ethanol vapor the decomposition rate is proportional to the ethanol vapor concentration whereas at high concentrations the rate becomes independent of the ethanol vapor concentration. A. Write a three-step mechanism for the ethanol decomposition process. Use for your notation: A = ethanol; S = available catalytic sites on the copper surface; AS = ethanol - surface site complex; B = products. B. Derive from this mechanism the rate law for the decomposition of ethanol on a hot copper surface and show how it can account for the observations mentioned above concerning the dependence of the decomposition rate on ethanol vapor concentration. In addition to the steady-state approximation, you will want to introduce a constraint that arises because the total number S0 of catalytic sites is fixed. and since the total number of suface sites doesn’t change:
Therefore: at low [A] --> k1[A] is negligible rxn is 1st order in [A] at high [A] --> k1[A] dominates rxn is 0 order in [A] |