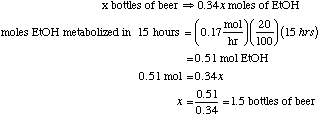Kinetics Solutions: #4
4.* (1995 F 9) A worker in a dry cleaning plant is exposed through inhalation to halogenated hydrocarbons that bind strongly to the same liver enzyme (alcohol dehydrogenase) that catalyzes the oxidation of ethanol (C2H5OH) to acetaldehyde (CH3CHO). These halohydrocarbons persist in the liver for several days. Suppose one evening this worker walks out of the plant with 80% of the liver enzyme sites occupied by halohydrocarbons and is invited by a friend to "have a few beers" before dinner. Please recall the homework problem on hyman alcoholysis in which you learned that the zero-order rate constant for oxidation of ethanol in a fully functioning liver is 0.17 mole/hr and that one bottle of ber contains 0.34 moles of ethanol. Provide this worker with an estimate of how many bottles of beer he can drink and be fully sober (free of ethanol) by the start of the next morning (15 hours later).
|