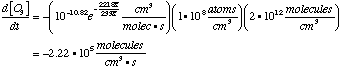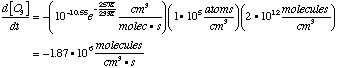Kinetics Solutions: #3
3.* (1996 F 16) At an altitude of 30 km and a latitude of 00 the atmospheric temperature is 233 K. The concentration of ozone and oxygen atoms are chlorine atoms are 2 x 1012 molecules/cm3, 1 x 108 atoms/cm3, and 1 x 105 atoms/cm3, respectively. Under these conditions the following reactions occur, each of which obey the Arrhenius rate equation: k=Aexp(-Ea/RT):
Where the units are cm3molecule-1s-1for A and K for Ea/R.
A. Write down a differential equation for the disappearance of ozone from the first reaction. These are all elementary reactions, so back reactions may be ignored.
B. Find the rate for ozone dissapearance from reaction 1. From A, We also know that Therefore,
C. Write down a differential equation for the disappearance of ozone from the second reaction.
D. Find the rate for ozone disappearance from the second reaction.
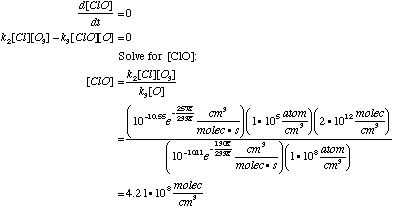
E. Use the information you have to estimate the steady-state concentration of ClO. From reaction (2), From reaction (3), The total rate of change in [ClO] is the sum of these:
We are to estimate the steady-state concentration, so:
F. What is the role of Cl in the second and third reactions Cl is a catalyst. G. How does the rate of destruction of ozone from the first reaction compare to the rate of destruction from the second and third reactions? Rate from (1) is Rate from (2) and (3) is Thus the rate of (2) and (3) is 8.4 times faster than the rate of (1). This is why CFC's are bad.
|