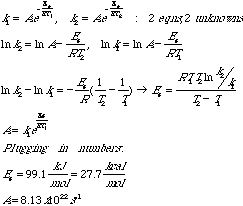Kinetics Solutions: #2
2.* (1997 3 5) The gas reaction N2O5 --> 2NO2 + 1/2O2 follows the Arrhenius equation k = Aexp(-Ea/RT) where R = 8.314J K-1 mol-1 and 1 calorie = 4.184J. At the temperature T=273K, k=0.0887 x 10-5 s-1, whereas at T = 298K, k = 3.46 x 10-5 s-1. What are the values of the activation energy Ea in kcal/mol and the pre-exponential factor A is s-1?
|