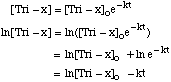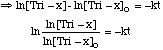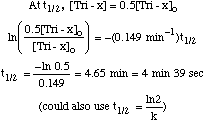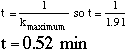Kinetics Solutions: #11
11.* (1993 3 4) The development of a photographic mage on film is a process controlled by kinetics. Exposed silver halide crystals in the film are reduced to silver in a developing solution. The development process is performed for a certain amount of time, which depends on the temperature of the developing solution, and with agitation to ensure transfer of reagents to the film. A. The time required for development at a particular temperature is inversely proportional to the rate constant for the process (assume k = 1/t). The following table gives the development times (in minutes) for Eastman Kodak’s Tri-x film in two types of developing solution.
Use the Arrhenius equation to dtermine the activation energy (in Kcal/mol) for the development process using D-76 developer. (R = 1.986 cal mol-1 K-1) Arrhenius Equation: since which is of the form y = mx + b, so plot ln 1/t v. 1/T (in Kelvin!) Plot on graph paper, estimate straight line: slope and y-intercept
Data (D-76)
Ea = 14,860 cal / mol = 14.9 kcal / mole [full credit for anything between 7.44 and 19.86] B. Film development is a first order process, and for Tri-x is 45% complete after 4 min. at 20šC using D-76 developing solution. What is the rate constant for this process, and what is the half life? For a 1st order process,
If 45% complete, then [Tri-x] = 0.55[Tri-x]o. So, for t = 4 min,
C. Increasing the temperature speeds the rate at which the film is developed. There are, of course, limits to the temperatures which can be used. For example, at elevated temperatures the emulsion on the film begins to degrade, resulting in a "grainy" picture. The maximum temperature of development is defined as the temperature at which the rate of degradation exceeds the rate of development. For Tri-x film the activation energy for the emulsion degradation process is 19.86 Kcal/mol with a preexponential factor of 3 x 1013 s-1. Which developer (T-max or D-76) will allow us to develop Tri-x film the most rapidly? Explain. Given: From part (a) find A (pre-exponential factor) from y intercept => ln A=23.45 so A = e23.45=1.53•1010. Now we know: set kdeg = kT-max, solve for T => T = 312 K. At T = 312, kT-max = 0.35 set kdeg = kD76, get T = 329 K. At T = 329, kD76 = 1.91 Therefore, D-76 can be used at higher T where it is much faster than T-max. D. What is the time? (Hint: use your graph from part 4A)
|