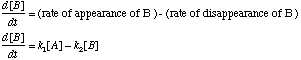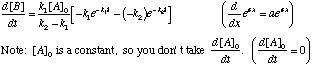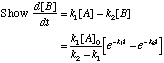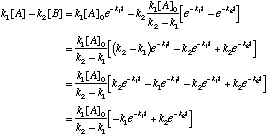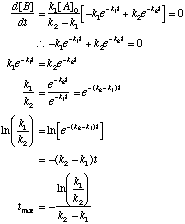Kinetics Solutions: #10
10.* (1994 3 7) Consider two consecutive first-order reactions
in which the reactant A is transformed into the product C via the intermediate B. Many examples of this behavior are found in radioactive decay processes in which the intermediate is also radioactive (unstable). The rate equation for the disappearance of the reactant A is
The rate equation for the appearance of the product C is
A. Write down an expression for the time rate of change of the concentration of
B. Differentiate
First, differentiate with respect to time:
Now, try to equate your answer from part A to this expression. (This is much easier than simplifying the above expression.)
Recall that Also note from above that, as given in part B,
Substituting:
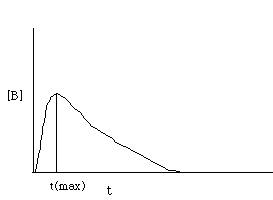
This last statement completes the problem. C. How does the [B] vary with time? Under what conditions does [B] show a maximum value? There are two cases to consider: If k1>k2, the concentration of B will build up very rapidly and then decay slowly over time. Essentially,
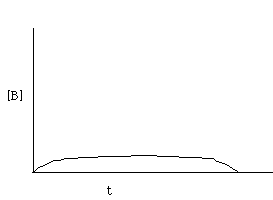
For k2 > k1, the concentration of B will never build over time. As soon as B is formed, it will decay to give C. This looks like:
To find the maximum value, take the derivative and set it equal to 0:
This value of t can be substituted into the equation for [B] to give [B]max. |