Group Theory Solutions: #9
9.* (1993 1 1) Allene (1,2-propadiene) has the formula C3H4 and consists of three carbon atoms connected by double bonds and two pairs of terminal H atoms: CH2=C=CH2. A. Draw a picture looking down the C-C-C axis showing the orientation of the two filled pi orbitals.
B. Are the two CH2 planes parallel or perpedicular to one another? perpendicular C. Name each symmetry element present for allene. Name each element as many times as different kinds of the same type exist in the molecule, e.g. if there are two different (noncoinciding) C2 rotation axes, name C2 twice. Do not guess; wrong answers will be subtracted from right answers. Allene has E, S1, 3C2, and S4. D. What is the formal charge on the central carbon atom in allene? The formal charge on the central carbon atom is (4 electrons - 4 bonds - 0 nonbonded = 0 E. Is the allene molecule polar? Explain. Polarity is limited by symmetry considerations; only Cn, Cnv, and Cs molecules can be polar and allene is D2d. Allene has elements of symmetry which are perpendicular. Another way to see it is that the "polarity" (dipole moment) vectors cancel out.
|
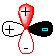
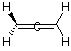
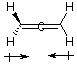 The resultant polarity vector
is 0.
The resultant polarity vector
is 0.