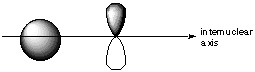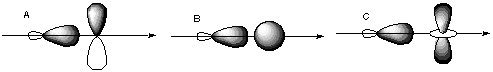Group Theory Solutions: #77. A good rule of thumb is that bonds can only form when both participating orbirtals have the same inversion symmetry about the internuclear axis. A. In what relative positions must an s and p orbital be in order to bond? What is the symmetry of each about the internuclear axis?
Both the s and the p orbital have C-infinity-v symmetry about the internuclear axis. B. In what relative positions would s and p orbitals have cancelling overlap?
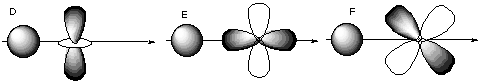
Here, the s orbital has C-infinity-v symmetry about the internuclear axis, but the p orbital has C2 antisymmetry about the internuclear axis. C. Which of the following orbitals could form bonds with each other?
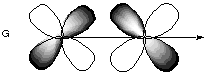
The only pairs of orbitals with non-cancelling overlap are B, E, and G. These orbitals all have the same inversion symmetry about the internuclear axis.
|