Group Theory Solutions: #5
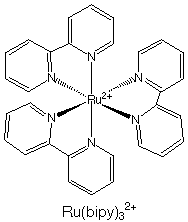
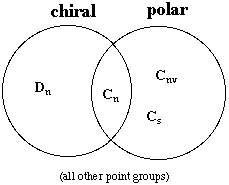
5.* (1995 1 4) Are all chiral molecules polar? Are all polar molecules chiral? What are the conditions for a molecule to be nonpolar? What are the conditions for a molecule to be achiral? •The only molecules that are polar are those in the Cn, Cnv, and Cs point groups. For a molecule to be nonpolar, it must not be in the Cn, Cnv, or Cs point groups. •A molecule is chiral if it does not have an Sn axis. Therefore, the only chiral point groups are Cn and Dn. All other groups are achiral. •Not all chiral molecules are polar. Molecules in the Dn point group are chiral but not polar. An example is Ru(bipy)32+ (D3)
Not all polar molecules are chiral. Molecules in the Cnv and Cs point groups are polar but not chiral. An example is water (C2v). A summary of the situation can be diagrammed as follows:
|