Group Theory Solutions: #2
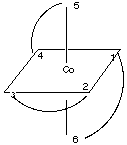
2.* (1997 F 12) The compound tris (ethylenediamine) cobalt (III), Co (H2NCH2CH2NH2) 33-, has the structure pictured below:
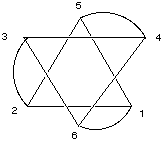
where the curved lines represent the three bidentate en ligands, each one of which is attached to the Co atom by the N atoms at two points. Another way to view this stucture is to realize that vertices (1,2,5) and (3,4,6) form triangles (planes) in which 1 is opposite 3, 2 is opposite 4, and 5 is opposite 6:
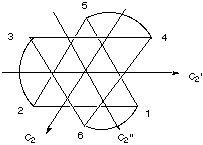
Answer the following questions about this molecule: A. Does this molecule possess one or more C2 axes of rotation? If so, where are they and how many are there?
B. Does this molecule possess one or more C3 axes of rotation? If so, where are they and how many are there? One C3 axis. It goes directly into the center of one of the 8 faces of the "octahedron" and comes out the other side. (This isn't an octahedron; it's D3, so it doesn't have the other C3 axes that an octahedron has. You can visualize the D3 point group by stretching or compressing the octahedron along one of its C3 axes. Stretching or compressing in this way breaks the other C3 axes of the octahedron, just as adding the bidentate ligands does.) C. Does this molecule possess an inversion center? no D. Does this molecule possess one or more reflection planes? If so, where are they and how many are there? no E. Is this molecule polar? Explain your answer. not polar; all dipole moment vectors cancel out. Another way to see this is that rotation symmetry about each C2 axis kills any dipole moment. F. Is this molecule chiral? Explain your answer. yes! This molecule is in the D3 group, which has no improper rotation axes such as i or s.
|