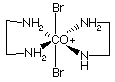Coordination Chemistry Solutions: #9
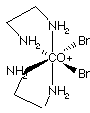
9.* (1996 2 3) Using only ethylene diamine (en = H2NCH2CH2NH2) and bromide anions as ligands construct a cationic octahedral complex of cobalt (III); your complex cation should have charge +1 and it should be chiral. Draw a three dimensional structure for this coordination complex below on the left. Then draw the structure of a diastereoisomer on the right. The chiral cation and its diastereomer are shown below. The diastereomer is not optically active because it has an internal mirror plane, S1= s.
|