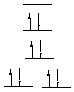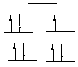Coordination Chemistry Solutions: #8
8.* (1996 2 1) Consider the following compounds; indicate the number of unpaired electrons in each. A. [Pd(NH3)4]2+ NH3 is a neutral 2 electron donor. Therefore, we're looking at a d8 complex of Pd(II), which is square planar. There are no unpaired electrons:
C. [Co(CN)5]3- CN- is a strong field ligand (due to its conveniently situated p* antibonding orbitals), so the complex is low spin. This is a Co(II) complex, which is d7. Because there are 5 ligands, the shape of the complex is trigonal bipyramidal. There is 1 unpaired electron:
|