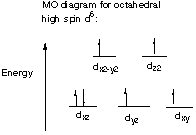Coordination Chemistry Solutions: #7
7.* (1996 F 13) Below are structural formulas for two iron porphyrins. The pyridine complex, X, is paramagnetic whereas the carbonyl derivative is diamagnetic. The pyridine complex strongly binds a second pyridine forming a diamagnetic bis-pyridine complex; however, the carbonyl complex, Y, only weakly binds a second carbonyl.
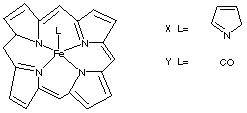
A. Offer two reasons for this difference.
- Backbonding: The carbonyl has antibonding pi orbitals that are optimally situated for backbonding. The Fe dxz orbital (or dyz, depending on how you draw it) can donate electron density into C-O p*, thereby stabilizing itself and increasing DO to make the complex go low-spin. Although this weakens the C-O p bond, it strengthens the Fe-CO bond and makes less Fe dxz electron density available for backbonding to the second CO. Hence the second CO is less tightly bound than the first. The pyridine, on the other hand, has orbitals less optimally situated for backbonding. Therefore it is high spin and the binding of the second pyridine, since it does not disrupt any stabilizing interactions, is strong.
- We are told in the problem that adding the second pyridine causes the complex to be diamagnetic, which means that all 6 of the Fe d electrons go into bonding t2g orbitals, causing the complex to become more stable.
B. How many unpaired electrons are in the mono pyridine complex?
The mono-pyridine complex is a high-spin d6 complex that has 4 unpaired electrons.
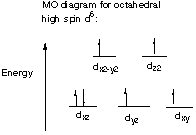
|