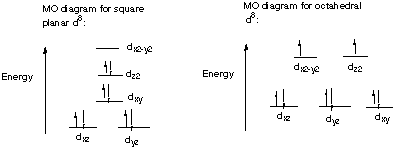Coordination Chemistry Solutions: #5
5.* (1996 F 2) Platinum II typically forms diamagnetic, square-planar complexes. Attempts to make octahedral Pt(II) complexes, even with a rigid multicyclic ligand which would force six nitrogens to bind in an octahedral fashion to Pt(II) have not succeeded even though such "sepulchrate" complexes of Co(III) are well known and are very stable. Use ligand field diagrams to explain the reluctance of platinum (II) to form octahedral complexes. Pt(II) is d8. Therefore, in an octahedral scheme it would have two electrons in antibonding orbitals. In the diagmagnetic square planar configuration, however, the highest energy dx2-y2 orbital is empty, so that the net energy is lower than for the octahedron. Co(III) is d6, so in an octahedral complex its antibonding orbitals are empty.
|