Coordination Chemistry Solutions: #2
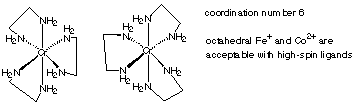
2.* (1997 2 2) A. Using any bidentate ("chelating") ligand of your choice, draw a structural formula for a first-row coordination complex which is both chrial and has three unpaired electrons. Also indicate the oxidation state, coordination number, and d electron count of the metal in your complex. Your structure should illustrate the chiral nature of this compound.
B. Identify the 3d metal in the following diamagnetic complex anion: M(CN)64-. What is the oxidation state and d-electron count for your metal?
|