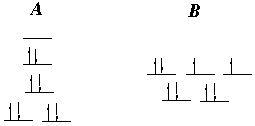Coordination Chemistry Solutions: #21
21.* (1992 2 4) Consider two complexes A and B. One is Ni(NH3) 2Br2; the other is Pd(OH2) 2Br2. A is paramagnetic and B is diamagnetic. A. What is the oxidation state of each of the metals? Each of the metals has a +2 charge to counter the 2 Br- ligands, so the complexes are Ni(II) and Pd(II). B. How many d electrons does each metal have? Both Ni and Pd are d10 in their standard metallic states, but removing 2 electrons from them makes them both d8. C. What is the coordination number of each complex? Each of our four ligands forms one bond. So, the coordination number is 4. D. What is the geometry of each complex? The different magnetic properties suggest that the two complexes have different geometries. A is a square planar complex. It has no unpaired electrons and is diamagnetic. B is a tetrahedral complex. It has two unpaired electrons and is paramagnetic.
E. Which is which? We are forced to decide which d8 complex will be tetrahedral. The ligands around the smaller nickel atom are more crowded and would probably prefer to be in the tetrahedral geometry. Therefore, A has Pd (2nd row, low spin), and B has Ni (1st row, high spin.) F. Identify the stereoisomers (if any) of each complex. A has two achiral stereoisomers (cis and trans.) B has no stereoisomers.
|