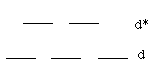Coordination Chemistry Solutions: #19
19.* (1993 2 3A) Which of the following is rouge and which is colorless? TiO2 and Fe2O3
Color in transition metal complexes is usually the result of d–d* transitions:
TiO2 has Ti4+. It is therefore d0. Because it has no d electrons, there can be no d–d* transitions, and TiO2 is colorless. (In fact, it's actually used as a white pigment in paint, toothpaste, and sunscreen.) Fe2O3 has Fe3+. It's d5. Because it has d electrons, it can undergo d–d* transitions, and is rouge. It's a component of rust. |