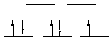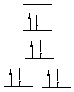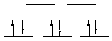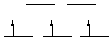Coordination Chemistry Solutions: #18
18.* (1993 2 1) For each of the following complexes indicate the oxidation state of the central metal, the d electron configuration and the number of unpaired electrons, and whether the structure is chiral or achiral. A. Fe(CN)63- Fe(III), d5, 1 unpaired electron, achiral B. Ru(bipy)33+ Ru (III), d5, 1 unpaired electron, chiral
strong field, low spin octahedral C. Pt(CN)42- Pt(II), d8, 0 unpaired electrons, achiral
square planar D. p-C6H5Mn(CO)3 Mn(I), d6, 0 unpaired electrons, achiral
strong field, low spin octahedral E. Ni(PPh3)2(CO)2 Ni(0), d10, 0 unpaired electrons, achiral (tetrahedral). F. Cr(acac)3 Cr(III), d3, 3 unpaired electrons, chiral
octahedral |