Coordination Chemistry Solutions: #17
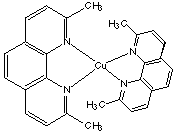
17.* (1993 F 5) 2,9-dimethyl orthophenanthroline is a reagent used for the selective binding of copper in one of its oxidation states. Which oxidation state of copper does this reagent favor? A copper complex of this ligand is shown below but the charge (and thus oxidation state) is not specified. Discuss this situation in the context of electronic structure and preferred coordination geometry.
Because the ligands on copper are so bulky, the methyl groups will clash with each other in a square planar configuration. Therefore, this complex prefers a tetrahedral configuration. Common oxidation states for the copper atom are Cu(I) and Cu(II). Cu(0) is d11, Cu(I) is d10 and Cu(II) is d9. Because d10 complexes with 4 ligands (or 2 bidentate ligands) prefer tetrahedral geometries while d9 complexes prefer square planar geometries, this complex is tetrahedral d10 with Cu(I). |