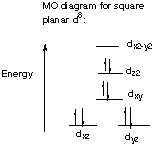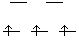Coordination Chemistry Solutions: #13
13.* (1994 F 7) A novel organometallic anion [Cu(CF3)4]- has recently been prepared. A. What is the shape of this anion? Explain your reasoning. Do you expect this substance to be diamagnetic? Each CF3 has a -1 charge, so CuIII is d8, square planar.
B. Calculate the oxidation state, the d electron configuration and the number of unpaired electrons in [N(Et)4]4[V(CN)6] (where Et = C2H5). NEt4 has +1 charge, so [V(CN)6]4- => V2+ = oxidation state is +2, so the complex is d3, which means that it is octahedral with 3 unpaired electrons in the t2g orbitals.
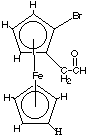
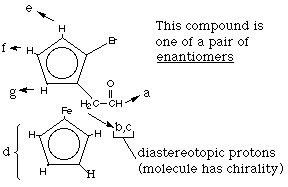
C. Consider the following ferrocene derivative… What sort of stereoisomers would this substance have? How many stereoisomers would this substance have? How many different 1H NMR signals do you expect? Identify each by marking on the structure.
7 different types of protons --> 7 1H NMR signals |