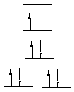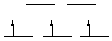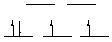Coordination Chemistry Solutions: #11
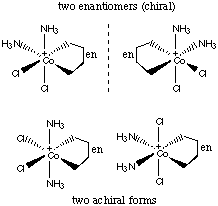
11.* (1995 2 2) A. Draw all possible stereoisomers of [CoCl2(en)(NH3)2]+. Which are chiral? (en = H2NCH2CH2NH2)
C. How many unpaired electrons are in the following complexes (assuming all of them have strong-field ligands?): Cu(NH3)42+, CrCl3(THF)3, K3Mn(CN)6. Cu(NH3)42+: Cu(II), d9, square planar, 1 unpaired electron
CrCl3(THF)3: Cr(III) is d3, octahedral, 3 unpaired electrons
K3Mn(CN)6: Mn(III) is d4, octahedral, 2 unpaired electrons
|