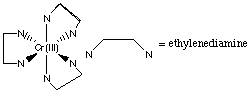Coordination Chemistry Solutions: #10
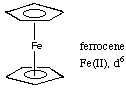
10.* (1995 F 1) E. Give an example of a ligand which has neither p electrons nor non-bonded electron pairs. H, CH3, H2 F. Give an example of a metal complex made only from an anionic aromatic ligand, show the structure, indicate the oxidation state of the metal and the d-electron count.
H. Draw a stereochemical structure for a coordination complex which is chiral and has three unpaired electrons.
I. Identify the 4d metal in the following diamagnetic complex: M(CO)6. Give its d-electron configuration. Mo(0) is d6. We know that the metal is uncharged because the net complex is uncharged. |