NMR Part 2 Solutions: #9
9.* (1993 F 3) A quote from the San Francisco Chronicle 11/25/93 "The traditional Thanksgiving menu contains roast turkey with all the trimmings--nuts, cranberry sauce, sweet potatoes and pumpkin pie--and a dash of aflatoxins, nitrates and malonaldehyde". The article goes on to say that several toxins which have been found to cause cancer occur in minuscule, harmless amounts in such a meal. If we treat our holiday dinner the same way we do Alar and other pesticide residues, the Thanksgiving meal would be banned. It makes more of a difference how much you eat than what you eat; eating 3.8 tons of such a meal could mutate your genes. Some people strongly disagree with this viewpoint. Among the toxins mentioned were: aflatoxin (from mixed nuts), eugenol (in the cranberry sauce) and malonaldehyde (from the turkey). A structural formula for eugenol is shown below.
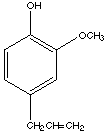 A. What is the origin of the suffix of this name? The "-ol" ending comes from alcohol. B. How many stereoisomers exist for eugenol? An answer of 1 (meaning eugenol has only 1 form) or 0 (meaning eugenol has no stereoisomers) was accepted. C. How many different 13C NMR signals would you expect in the 13C NMR spectrum of eugenol (ignore spin coupling)? There are 10 different carbons, and 10 different signals. (All 6 carbons on the benzene ring are different, as are all the carbons on the substitutents.) D. How many sites of unsaturation are in eugenol? 4 olefins + 1 ring = 5 or
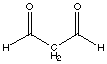
Malonaldehyde is also called "propandial". E. Write a structural formula for malonaldehyde.
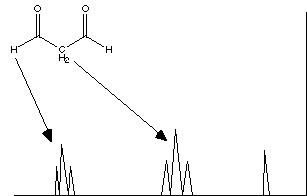
malonaldehyde (propanedial) F. Sketch the expected proton NMR spectrum for malonaldehyde; include relative chemical shifts from tetramethylsilane (show its signal) and indicate the spin coupling pattern. Assign the peaks to each type of protons.
Note the TMS peak on the far right. The carcinogen formaldehyde, CH2O, is also in the apples (derived from the apple skin). G. Sketch the expected proton NMR spectrum of monodeuteroformaldehyde, CHDO, show the spin coupling. Hint: D has a nuclear spin of 1.
Although deuterium does not show up on 1H NMR, it does affect the spectrum because its nuclear spin is 1. Therefore, its multiplicity is 2(1) + 1=3, indicating a triplet. It splits the hydrogen signal into 3 different, equally sized peaks. |