NMR Part 2 Solutions: #8
8.* (1994 2 2) Three regioisomers ("constitutional isomers") X, Y, Z of an aromatic hydrocarbon have the molecular formula: C8H10. The 1H NMR spectrum of hydrocarbon X exhibits two sharp singlets in a ratio of 2:3 (integrated intensities) (no spin-coupling was observed). The 13C NMR spectrum of X shows three singlets (ignore possible spin-coupling with H). Similarly, the NMR spectra of Y and Z show the pattern of H and C signals given below. The ratios of integrated intensities for the H signals are given in parenthesis.
|
Compound |
Number of H signals (ratios) |
Number of C signals |
|
X |
2 (2:3) |
3 |
|
Y |
3 (3:1:1) |
4 |
|
Z |
4 (6:2:1:1) |
5 |
On the basis of these data, write structural formulas for X, Y, and Z.
Note that C8H10 has 4 sites of unsaturation, and we know from the problem statement that it’s aromatic, so since there are 8 C’s there must be a benzene ring in there somewhere.
|
X:
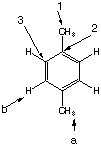
|
All aromatic protons (a) are identical and will only show one peak which is not split. The methyl hydrogens (b) are all identical and the protons will not be split by protons (a) since they are more than 3 bonds away. The 2:3 ratio stems from the actual 4:6 ratio of aromatic to methyl hydrogens. There are three types of carbons, the methyl carbons (#1), the aromatic carbons directly bonding to the methyls (#2) and the aromatic carbons bonding to hydrogens. (#3). X is para-xylene.
|
|
Y:
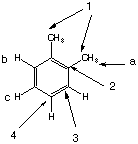
|
There are three types of protons in this molecule, two types of aromatic protons (b,c) and the methyl protons (a). They are in a ratio of 6:2:2 for a:b:c. There are also 4 different types of carbons as labeled on the diagram. Y is ortho-xylene. |
|
Z:
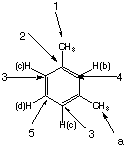
|
There are nwo 4 different types of protons, the methyl protons (a) and three different types of aromatic protons (b, c, d.) There are 5 different carbons (1-5). Z is meta-xylene. |
|


