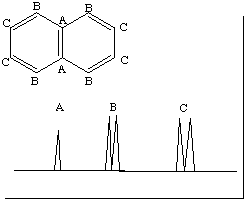
NMR Part 2 Solutions: #1111.* (1990 F 3) Explain what the 1H and 13C NMR spectra of napthalene would look like. In the 13C NMR consider only the coupling between adjacent atoms. In the 1H NMR consider coupling through no more than three bonds. 13C NMR (proton coupled) There are 2 carbons A with no adjacent hydrogens. These are singlets. B and C are both doublets because they have 1 adjacent hydrogen. There are two of each type of carbon. Typically you cannot experimentally deduce integration from 13C NMR, so the relative intensity of the peaks you draw is not important.
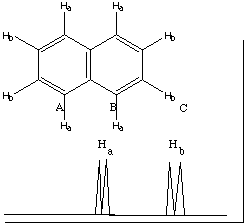
 1H NMR There are two hydrogens Ha, each of which are split by one H b proton into a doublet. There are 2 hydrogens Hb, each of which are split by one Ha proton into a doublet. Note that equivalent hydrogens do not split each other.
|