NMR Part 2 Solutions: #10
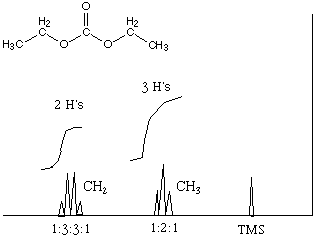
10.* (1993 2 4) A. Sketch the 1H NMR spectrum you expect for diethylcarbonate. Show spin coupling patterns, including the expected ratio of peak heights.
J (the spacing between the peaks or the coupling constant) is the same for both the quartet and the triplet. B. How would the spectrum be different with a spectrometer which had twice the magnetic field strength? Chemical shift is defined as follows: d = (n - nref) / nspec, where d is the chemical shift and the n's are the frequencies of the sample's resonance, the reference (usually TMS), and the spectrometer, respectively. Increasing the frequency of the spectrometer proportionally increases the frequency at which resonance occurs for the sample peaks and the reference. This gives a greater separation of peaks when they are plotted versus frequency, making it easier to tell them apart. For this reason, chemists always want more expensive, high-frequency spectrometers. However, when the peaks are plotted versus chemical shift, the increase in freqency of the sample peaks cancels upon being divided by the increase in frequency of the spectrometer. This is why chemical shift is independent of the frequency of the spectrometer. Although higher frequency spectrometers will give a cleaner spectrum when plotted versus chemical shift for the reason stated above, they will not give a spectrum that has different chemical shift values for the peaks.J is the coupling constant between the atoms that split each others' peaks. It is usually given in Hz because the energy of the splitting does not depend on the external magnetic field. So J (in Hz) is constant no matter what. Let's say that we have a peak whose center of resonance occurs at x in frequency. And lets say that it is split into two peaks by an adjacent hydrogen with coupling constant J. Then one of the peaks into which it is split will occur at x + J/2 and the other will occur at x - J/2. Now, the frequency of our spectrometer is vspec. Therefore, if we calculate the splitting between the two peaks in chemical shift, we obtain the following:
So while the splitting in frequency does not depend on the frequency of our spectrometer, the splitting in chemical shift does. If we were to double the frequency of the spectrometer, we could expect the distance between peaks J to be cut in half when the peaks are plotted versus chemical shift. |