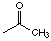
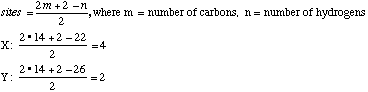NMR Part 1 Solutions: #7
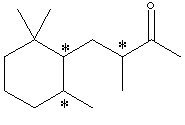
7.* (1994 F 2) The perfume industry can be traced back through many civilizations; for example, in the 5th centruy BC Herodotus included a chapter on perfumes in his "Histories." In modern times, some perfumes are made by synthetic organic chemists, while others are isolated from natural products. Consider a-isomethylionone, X, a component derived from violet flower oils. The structural formula of X is shown below in chichenwire notation.
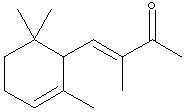
A sample X (Xn) derived from natural flower oils is optically active; however, a sample of X (Xs) produced from a total synthesis does not rotate plane-polarized light even though this synthetic sample, Xs, exhibits both an infrared spectrum and 1H and 13C-NMR spectra identical to those of the sample Xn derived from natural sources. The natural and synthetic samples Xn and Xs have different fragrances. Catalytic hydrogenation of one mole of either Xn or Xs over a Pd catalyst took up to 2 moles of H2 (as indicated below). The product Ys from Xs was shown to be a mixture of 4 components. When the 4 components of product Ys were separated with a gas chromatograph, each component showed the same molecular formula (C14H26O) as determined by mass spectrometry. Xn, Xs, and the 4 components of Ys show a strong infrared peak in the region associated with a ketone (1690-1700 cm-1). On the basis of this information answer the following: A. How many sites of unsaturation are in X and Y, respectively? use Or, you could count sites, including C=C bonds, C=O bonds, and rings. B. Write an asterisk beside each chiral center in the structural formula shown above for X.
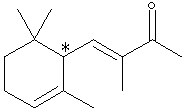
C. Identify the nature of the apparent stereoisomers: Xn and Xs. Xn is an optically pure chiral compound (one enantiomer.) Xs is a racemic mixture of Xn and its enantiomer. Synthesis with achiral reagents will always give optically inactive products. If the products are chiral, they will be racemates. Although the problem does not state whether the reagents were chiral, the fact that Xs and Xn have the same NMR and IR spectra means that Xs can’t just be a diastereomer of Xn. The fact that it is optically inactive means it’s not just an enantiomer of Xn. This leaves only a racemic mixture of Xn and its enantiomer. D. Write the structural formula for Y in chickenwire notation. see below E. Place asterisks beside each chiral center on your structure for Y.
F. What is the nature of the four stereoisomeric components represented by Y? A simple term will do. Gas chromatography does not separate enantiomers. Thus each component is a pair of enantiomers which is a diastereomer of each of the other pairs. G. Using the terms R and S, explain why Yn would exist as 4 stereoisomers. Yn should be the product of catalytic hydrogenation of Xn. Yn has 3 chiral carbons, but one of them will have the same absolute configuration as it did in Xn. The other 2 carbons will be evenly distributed in R and S, allowing for 4 diastereomers, each of which is one enantiomer of a pair whose other enantiomer is not produced: XRR, XRS, XSR, XSS. Also acceptable for full credit: Since stereoisomer has been used interchangeably with separated component in this problem, one may have interpreted the problem as asking why 4 components were separated from Ys. This is because Ys has 3 chiral carbons and thus 23=8 stereoisomers, but only 4 enantiomeric pairs: RS RS RS RS RS RS SR SR RS SR RS SR H. Now consider hydrogenation of Xs to form Ys. How many components would you expect to separate from the product Ys? Would any of these exhibit optical activity? Explain in two sentences. Hydrogenating Xs (racemic mixture) will produce 4 pairs of enantiomers. Standard separation technologies are achiral and 4 components (each a pair of enantiomers) could be separated. Each component would be optically inactive, as they are racemic mixtures. RRR / SSS RRS / SSR RSS / SRR RSR / SRS
I. How many methyl signals would you expect to see in the 13C NMR spectrum of Xs (ignore spin coupling).
5 methyl signals. Each is different! 1 and 2 are inequivalent because they are on different sides of the ring and adjacent to a chiral center, making them diastereotopic. J. Which of the methyl signals would resonate at lowest field (i.e. have the largest chemical shift)? 5 (Ketone methyl) since it is adjacent to the electron-withdrawing carbonyl group.
K. Could you separate Ys into even more components with a chromatography column; if so indicate a crucial property of such a column? Yes. Since each component is a racemic mixture, a CHIRAL column could be used to separate these. L. Do you expect the 4 components Ys to have different fragrances than Xn? Explain. Since the 4 components Ys are completely different compounds from
Xn, they will probbaly have different fragrances. |