NMR Part 1 Solutions: #6
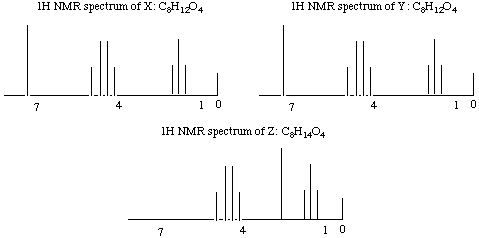
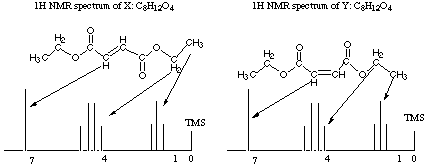
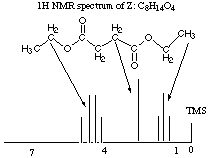
6.* (1995 2 1) Three 1H NMR spectra are displayed; X and Y represent substances having the molecular formula C8H12O4; Z has a formula C8H14O4. Reaction of either X or Y with H2 over a Pd metal catalyst forms Z. IR data shows that X, Y, and Z have one or more carbonyl-containing functional groups.
A. Propose structures for X, Y, and Z by writing out structural formulas on each spectrum; make assignments of each proton type to the 1H NMR signals. Assign the signal at 0 shown on each spectrum.
The first thing to think about with this as with any NMR question is sites of unsaturation: X and Y both have three and Z has 2. The second thing to note is that we get Z from X or Y after reaction over a Pd catalyst. Pd catalysts selectively wipe out C=C double bonds (and sometimes take out C=O double bonds too, but we know they didn't take out the C=O double bonds in this case because we are given in the question that there are carbonyl-containing functional groups in the product). The third thing to notice is that this is a highly symmetric molecule we are dealing with: there are only 3 kinds of protons (clusters of peaks) in X and Y and only 3 kinds of protons in Z! The fourth thing to notice is that X and Y have the same molecular formula and NMR spectra. They must be stereoisomers of each other. You might at first think they were enantiomers because enantiomers would show the same NMR spectra, but it would be difficult to get a chiral center out of so few different atoms, and we know that ethyl groups (see next paragraph) don't contain any chiral centers! X and Y could, however, be E and Z stereoisomers. The fifth thing to notice is that there's a triplet next to a quartet: dead ringer for an ethyl group!! Did you notice all that and write it down? Good, then you've already gotten a significant amount of partial credit no matter what you come up with for a structure. The true structures are shown above. Note that for simplicity, there is only one arrow to each cluster of peaks, though you know there should be two arrows to each!! B. Do you have sufficient information to distinguish X from Y? We don't have sufficient information to distinguish X from Y, though we know that the two molecules are E and Z stereoisomers. C. Deuterium has a spin of 1; sketch the 1H NMR spectrum of D-H and compare this with the spectrum of H-H. Deuterium has a spin of 1, so it has I = 2*1 + 1 (or 3) possible orientations in a magnetic field. Therefore, we would expect it to split the signal of its adjacent proton into three signals. Notice that deuterium itself does not show up on the 1H NMR spectrum because its resonance occurs off the scale of the 1H NMR experiment. Trying to put in some deuterium peaks was a common mistake on this exam as I recall. J Notice also that the three peaks into which the proton signal is split are the same size, as for problem #2 part E. |