NMR Part 1 Solutions: #2
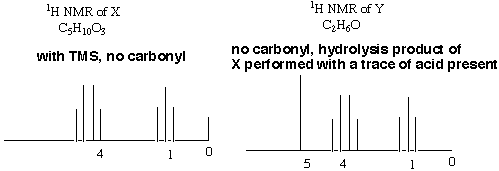
2.* (1997 2 1) Two 1H NMR spectra are shown, one of a substance X, C5H10O3 and another of its hydrolysis product Y, C2H6O. It is important to note that the spectrum of Y was performed with a trace of acid present. Furthermore, there is a small amount of TMS (tetramethylsilane) present in the spectrum of X but there was no TMS present in the spectrum of Y. The reaction converting X to Y is shown below as an unbalanced equation. A water soluble gas Z evolved during this reaction; a sample of Z showed no 1H NMR signal. The mass spectrum of Z revealed a parent ion at mass 44. The infrared spectrum of X showed a strong peak at about 1750 cm-1–a region associated with carbonyl groups in aldehydes, ketones and esters. No such peak was found in the infrared spectrum of Y.
On the basis of these data answer the following questions. A. How many sites of unsaturation in X, Y, and Z? X (C5H10O3) has 1 site of unsaturation. Remember that O's don't count in finding sites of unsaturation, and the general formula CxH2x+2 is zero sites of unsaturation, so getting rid of 2 H’s to create the general formula CxH2x leaves room for one double bond or one ring, i.e. one site of unsaturation. Y (C2H6O) has zero sites of unsaturation. We ignore the O's, so the molecule fits the general formula for zero sites of unsaturation, CxH2x+2. Since it has zero sites of unsaturation and has an O, we can be sure that the molecule has an alcohol functional group. Try to draw a molecule with that formula that does not have an alcohol functional group and you’ll see why it has to have one. To find the number of sites of unsaturation in Z, we first have to ascertain what Z is. We are told that it gives a mass spectrum peak at m/q = 44. From the unbalanced equation, we know that it must be composed of some combination of hydrogen, oxygen, and carbon. The only reasonable compound that fulfills all of these criteria is carbon dioxide, CO2. This molecule has 2 double bonds --> two sites of unsaturation. B. What alkyl group is represented by the 1H NMR spectrum of X? An ethyl group. The first thing you should note when you look at X’s NMR spectrum is that there must be a high degree of symmetry in the molecule, because there are 5 carbons and only two clusters of peaks! The fact that there are only two clusters of peaks means that there must be only two "types" of hydrogen atoms in the molecule. The downfield, deshielded (to the left) cluster represents the spin flip of a group of protons whose adjacent carbon has 3 protons, while the upfield, more shielded (to the right) cluster represents the spin flip of a group of protons whose adjacent carbon has two protons. Since there are no other groups of peaks, we must have 2 protons next to 3 protons and no other protons on the molecule. This is the situation for an ethyl group. (But wait a minute! There are 10 protons on the molecule total and this only accounts for 5! What happened to the other 5 protons?! From this we conclude that there are two ethyl groups on the molecule that are exactly symmetrical to each other so that they don't show up as separate peaks.) C. Deduce the structure of X from its 1H NMR spectrum. On the 1H NMR spectrum of X write its structure and indicate which peaks are associated with each type of 1H NMR signal. CH3CH2OCOOCH2CH3. This is the only way we can have the ethyl groups exactly symmetrical so that their peaks overlap. Note that there is one degree of unsaturation as we said in part A and that the protons nearer the O's flip first (are more downfield). 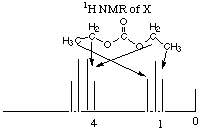 D. Assume the alkyl group in X is retained in the hydrolysis product Y. On the 1H NMR spectrum of Y write a structural formula for Y and assign the peaks to each type of 1H NMR signal. CH3CH2OH. Again, the quartet representing the two protons closest to the O is more downfield than the triplet representing the methyl H’s. By far the furthest downfield (most deshielded) is the alcohol proton. Note that if there were not acid present, this would not be a singlet but a triplet because it is adjacent to a carbon attached to two protons. Because there is acid present, the hydroxy proton is constantly coming off and on again, "exhanging" with other protons in solution. For this reason, the peak is a singlet. 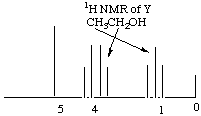 E. How many 13C signals would you expect in the 13C NMR of X, if you ignore 1H-13C coupling? 3. There would be one for the two methyl carbons, which are in identical chemical environments and therefore do not generate separate signals. There would be another signal for the two carbons on either side of the carbonyl carbon in the middle. The third signal would be for the carbonyl carbon, which does not have any protons attached. F. Write a structural formula for Z. As in part A above, the formula for Z is CO2. 
G. What is the role of the trace of acid in the spectrum of Y? Explain in 2-3 sentences. As described above in the answer to part D, the acid changes what would have been the triplet signal of the hydroxy proton into a singlet by facilitating rapid proton exhange in the solution. |