NMR Part 1 Solutions: #1
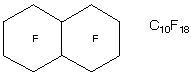
1.* (1997 F 3) Perfluorocarbons are hydrocarbon derivatives in which every hydrogen atom has been substituted by a fluorine atome. Perfluorocarbons have many uses. For example, these liquids can dissolve large amounts of molecular oxygen and allow patients to breath oxygen through lungs full of liquid rather than gaseous air. Consider perfluorodecalin, whose structure was represented as shown below in a recent article (Chemistry in Britain, August 1997).
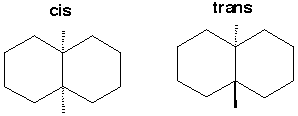
A. Suppose you employed a simple gas chromatograph to anlyze perfluorodecalin. How many stereoisomers would you expect to find? Indicate the nature of these stereoisomers.
2 achiral stereoisomers: cis and trans B. 19F (100% natural abundance) has a nuclear spin of plus or minus 1/2 (like the proton). Explain the use of 19F NMR to analyze a sample of perfluorodecalin; explain the number of 19F resonances youwould expect to find in an unpurified sample (ignore spin coupling). For the cis you would expect 5 19F resonances. For the trans you would also expect 5 19F resonances. Since the cis and trans forms are diastereomers, the resonances would occur in different places for these 2 isomers, so that NMR would be a good way to tell whether your sample were stereoisomerically pure. |